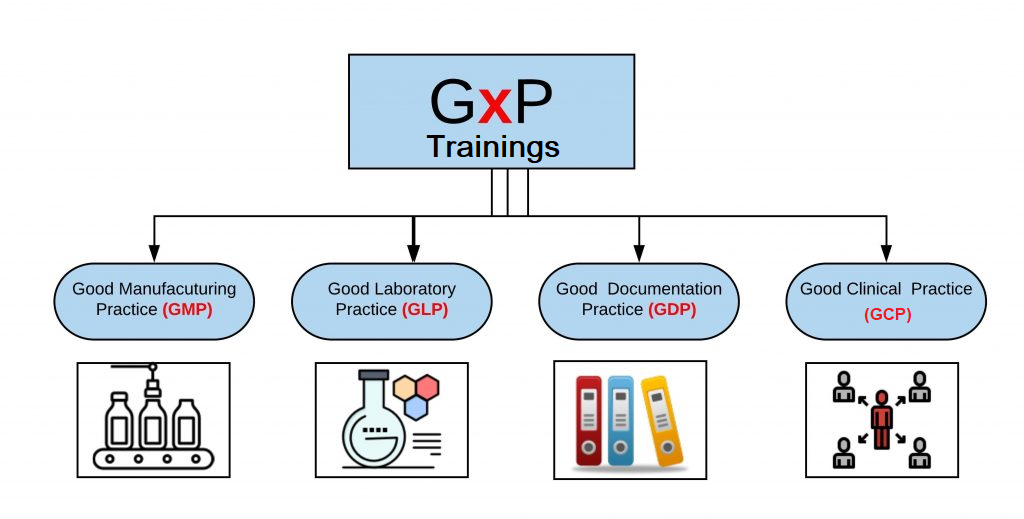
GxP is a set of regulations and quality guidelines formulated to ensure the safety of life sciences products while maintaining the quality of processes throughout every stage of manufacturing, control, storage, and distribution. The GxP standards were established by the Food and Drug Administration for a range of compliance-related activities and are recognized as:
G: Stands for good
x: Variable
P: Stands for practices
The variable “x” depends on the application of the standards. The value of x can be M for “Manufacturing”, C for “Clinical”, L for “Laboratory”, S for “Storage”, D for “Distribution”, R for “Review”, etc. The purpose of the guidelines is to ensure that the regulated organizations comply with the standard processes of various functions. GxPs are mostly similar across all countries.
The guidelines mainly focus on the following areas:
- Traceability– ensuring that the development history of the product can be reverse-engineered.
- Accountability– Identifying the contribution of every individual involved in the development process.
- Data Integrity– Ensuring the reliability of data.
Why is GxP important?
Since the regulations of GxP are global, every company manufacturing life sciences products is affected by it. Therefore, meeting the GxP requirements is highly important. Though there are several GxPs, few of them are highly important for the life cycle of any product.
- Good Manufacturing Practices (GMP)– GMP are the guidelines recommended by agencies for the authorization and control of manufacturing of products such as drugs, medical devices, active pharmaceutical ingredients (APIs) etc. Adhering to these guidelines assures the agencies about the quality of the products and that the manufacturers have taken every possible measure to ensure the safety of the product.
- Good Clinical Practices (GCP) – GCP is an international quality standard defined by the International Conference on Harmonization (ICH) that state the clinical trial regulations for the products that require testing on human subjects. The standards outline the requirements of a clinical trial and the roles and responsibilities of the officials involved in it. It ensures that no human experiments are performed just for the sake of medical advancement.
- Good Laboratory Practices (GLP) – These are the standards set by the FDA for non-clinical laboratory tests and studies conducted for assessing the safety and efficacy of the product. GLPs are a set of standards that define the framework for a non-clinical study and state how they should be performed, evaluated, reported, etc.